In our Cultinews of August 2019, we already published a first version of this work. Now, we will expand the information captured then along with a review of the factors that influence lipogenesis, or what is the same, in the biosynthesis and accumulation of oil within the fruit.
INTRODUCTION
From the fertilization of the “rapa” (olive flower) and until the olive reaches its maturity, the fruit goes through a series of stages according to a precise and determined pace (Figure 1).
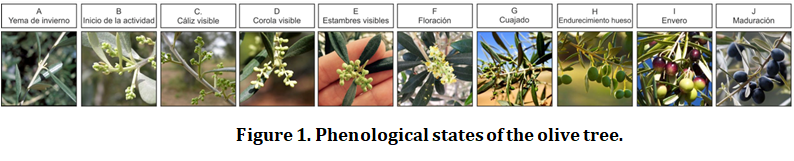
Pollination takes place during fertilization, this is the transfer of pollen from the anther of the flower to its stigma or from another flower. Usually, only one of the four seminal primordia of the ovary is fertilized and begins to grow. It has been observed in numerous olive varieties that cross-pollination anticipates the growth of fertilized seminal primordium compared to self-pollination, increasing the need for assimilates in these small fruits. This results in a strong competition between them and the flowers yet to fertilize, which turns out into a massive abscission of less competitive flowers and young fruits. This is the reason that the percentage of curdled flowers that give rise to fruit development is so low (1-2%) If you are interested in knowing how floral differentiation and induction occurs and what are the factors that affect and how, pollination and fruit set in olive trees, we invite you to consult our Cultinews of May 2020.
FRUIT GROWTH AND DEVELOPMENT
Quantitatively , the growth of the olive, like any other drupe, goes through several phases (Figure 2). In the first phase of growth, both cell division and expansion contribute to the increase in fruit size. This phase concludes around the end of sclerification or hardening of the bone (endocarp), which occurs between 7 and 9 weeks after flowering. After the period during which the growth slows or stops, a second phase begins where the olive experiences another increase in size, which concludes with the veraison or change in color (epidermis or epicarp) towards yellowish green tones that indicate the beginning of ripening.
The size of the fruit is a key component for the quality of the olive, especially the table olive. In the normal development of the growth, the load of the tree (number of olives), is possibly the driving factor of the size of the fruit under certain conditions of medium and crop. In other words, the greater the number of fruits, the smaller their size. Therefore, the size of the fruit is the main quality criterion in the table olive, just as the fat yield is for oil. The pulp/bone ratio, which is related to the size of the fruit, is a determining element of fat yield, since pulp oil represents more than 95% of the total olive (Rayo y Cuevas, 2017).

After fertilization, a rapid process of cell division occurs, although it is only after 10-15 days that this rapid cell growth is observable. During this phase I, the cell division of most of the tissues present in the olive ends. The tissue that shows a higher degree of development is the endocarp, which can reach up to 80% of the volume of the fruit, while the mesocarp or pulp and the exocarp (outermost part, increase in size less appreciably. The process continues until the month of July with the sclerification and hardening of the bone. Water stress during this period produces smaller stones (Lavee, 1986) that can give rise to fruits with abnormally high pulp / bone ratios and even, under conditions of high water stress , can compromise the viability of the fruit.
During phase II, fruit growth slows down, the embryo and endocarp reach their final size, ending the hardening of the pit. Phase III is characterized by a rapid growth of the fruit due to the widening of the mesocarp cells, which determines the final size of the fruit. During this phase, oil biosynthesis begins to occur and its accumulation in the parenchymal cells of the pulp (lipogenesis). The availability of water in this phase determines the final size of the fruit and its oil content, giving rise to smaller fruits and lower fat content under stress conditions. This phase ends at the beginning of autumn when the fruits undergo the first changes in their pigmentation. Coinciding with the change in the color of the fruit, the seed reaches maturity and has a high germination power, which is subsequently reduced when the fruits show black coloration (Rayo and Cuevas, 2017).
After phase III, the growth of the fruit and the accumulation of oil are significantly reduced, and the ripening processes are carried out. Once the pulp reaches the final size, it can show weight fluctuations as a result of fluctuations in its humidity due to environmental conditions (rainfall and frost regime).
LIPIDS, LIPOGENESIS AND CHANGES IN OIL COMPOSITION DURING THE FRUIT RIPENING PROCESS
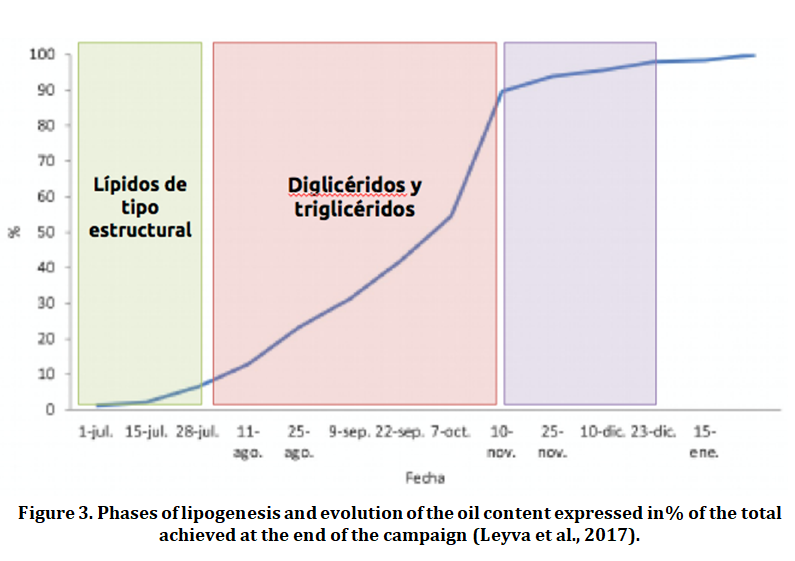
Synthesis of fatty acids (lipogenesis) in the pulp cells of the olive (mesocarp) determines the fat yield. The accumulation of lipids begins during the drupe growth stop phase and concludes at the beginning of ripening, in other words, lipogenesis takes place at the ending of the hardening of the bone to the veraison (envero) (Figure 3). These data seem to confirm previous studies on the fat yield of the olive that indicate that the amount of oil per olive reaches its maximum at the beginning of ripening and fluctuations from here are mainly due to variations in the humidity content of the pulp.

Three different phases can be established during the lipogenesis, (Frías et al., 1991):
- Slow biosynthesis phase. Occurs in newly formed fruits until the hardening of the bone and reaching a fat content of 4% in fresh weight. During this phase, the formation of structural lipids (phospholipids and galactolipids) takes place and the fruit behaves like a photosynthetic tissue.
- Accelerated biosynthesis phase. Occurs after the hardening of the bone, around the second half of July. An active synthesis of diglycerides and triglycerides initiates and will suffer a significant acceleration during the months of August (about 18 weeks after full flowering) and September, to reach its maximum towards late September or early October (García Martos y Mancha, 1992) overlapping with the change in pigmentation from green to yellowish green. At the end of this stage, the fat content of the fruit can reach up to 27% in fresh weight.
- Stationary or slowdown phase. In this phase, the speed of oil formation in the fruit begins to decrease progressively from mid-October until it disappears by early December, which corresponds to week 28-29 after flowering.
The maturation of the olive comprises a series of changes related to its compactness, color, sugar content, organic acids and taste factors that make it edible, regardless of the abscission or harvest (Figure 4). This is the result of a complex combination of physiological and biochemical routes, with a high genetic component, which can also be influenced by climatic and cultivation conditions (Beltrán et al., 2017).

Throughout the fruit ripening process, important changes are recorded in the fatty acid composition of the oil. Thus, the palmitic acid content falls, as does that of all saturated fatty acids. Oleic acid, the main fatty acid in olive oil (55-83%), shows a variable evolution since it can remain constant or show a slight increase in its content. As for linoleic acid, it increases its percentage throughout the fruit’s ripening process. In general, there is a tendency for fatty acid biosynthesis towards more unsaturated forms. An important parameter both from a nutritional and commercial point of view, mainly responsible for the oxidative stability of oils, is the ratio between monounsaturated and polyunsaturated acids (MUFAs / PUFAs); this ratio decreases during fruit ripening due to the increase in linoleic acid and the constant or slight increase in the oleic acid content.
An important fraction in virgin olive oil is that of minority compounds, since despite their low concentration they are of great importance due to their nutritional properties and their effect on the organoleptic characteristics of the oil. Due to their high antioxidant potential, phenolic compounds stand out, which are compounds that exert their antioxidant work at the cellular level, protecting the oil against autoxidation processes and, in addition, are responsible for some organoleptic characteristics of the oil (bitterness and spicy sensation). During the ripening of the fruit there is a decrease in its total content, as well as in general, of the different individual phenolic compounds, although sometimes slight increases in their concentration are seen as a consequence of the loss of moisture in the fruit caused by the Autumn frosts and their effect on the partition coefficients of these compounds.
Other natural antioxidants are tocopherols, which have vitamin E activity and also protect the body against oxidative processes. During the ripening of the fruit there is a decrease in the total content, as well as in that of each of the tocopherols present in the oil.
The sterols present in the oil constitute a parameter of regulated quality of virgin olive oil and in addition, they exert a healthy effect by reducing cholesterol levels in the blood. During the fruit ripening process there is a decrease in the total sterol content of the oil. Triterpenic acids and triterpenic alcohols have bioactive properties, protecting against oxidative stress and cell damage. In the case of acids, a decrease has been described during fruit ripening (Pérez Camino and Cert, 1999) while in the case of alcohols, no differences have been observed, although there is a tendency to increase their content (Sánchez et al. , 2004).
Oxidative stability is a measure of the oil’s resistance to rancidity. It depends on the acidic composition (MUFAs / PUFAs or oleic / linoleic) and the polyphenol content. Throughout the fruit ripening process, the stability of the oils decreases as a consequence of the increase in linoleic acid, and its effect on the relationships between fatty acids described above, and the decrease in the total polyphenol content.
The color of the oil is considered as an oil quality parameter and is related to its content and composition in pigments (Mínguez et al., 1991). The color of the oils varies from intense green to yellowish while it loses its intensity. As the fruit ripening process progresses, there is a decrease in the content of pigments, both chlorophyll and carotenoids, although chlorophyll decreases more rapidly, hence the relationship between carotenoids and chlorophyll shows an increase.
Another parameter that shows a decrease throughout the maturation of the fruit is the total content of sterols (Gutiérrez et al., 1999). Finally, the organoleptic characteristics of the oils are strongly influenced by the ripening state of the fruit, obtaining oils that are less bitter and with less marked sensory characteristics when ripening progresses.
FACTORS AFFECTING LIPID BIOSYNTHESIS
The biosynthesis and accumulation of oil in the mesocarp (pulp) of the olive is a process that is influenced by a series of internal and external factors, from the influence of the variety, since there are varieties with a greater potential in the accumulation of oil, to technical or design factors of the plantation, climatic factors and nutritional factors.
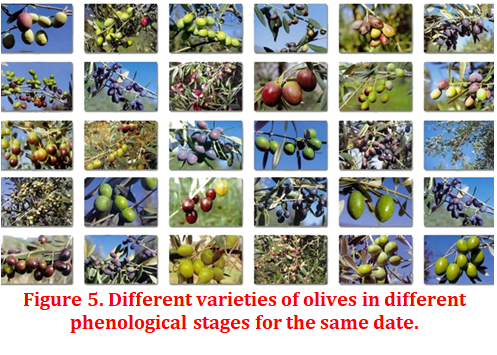
The influence of the genotype or variety (Figure 5) on the capacity for biosynthesis and accumulation of oil in the fruits is a very important factor, although it cannot be separated from the environment. In other words, the genotype-environment interaction is what really determines the ability of a variety to achieve a higher or lower fat yield. However, it is true that potentially the “Picual” variety is capable of accumulating a greater amount of oil (% on dry matter) than the “Arbequina” variety for the same environment. However, the biosynthesis and accumulation of oil in the fruit of the same variety does not have to be the same in one environment as in another. Therefore, the genotype is an important source of variation, not only of the main fatty acids, but also of the minority components of the oil, although its interaction with the environment is also significant, so part of the variability will depend on where a variety is planted. This must be considered when choosing varietals, since it could even have important commercial connotations (Navas López, 2019).
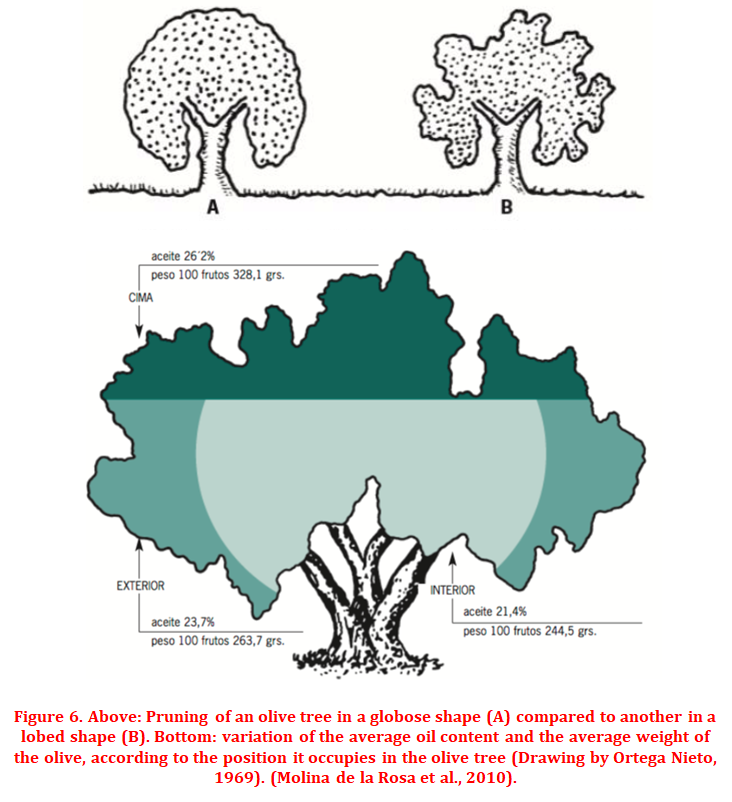
Within the influence of climatic factors, lighting (or radiation) and temperature vary jointly, so that more lighting means a higher incidence of radiation and, therefore, a higher temperature. The joint effect of both parameters translates into an increase in the enzymatic activity of different metabolic pathways (lipogenesis and phenolic synthesis). Thus, the brighter fruits reach a higher temperature and lower humidity, acquiring better maturity, more oil accumulation and more polyphenol content. However, an excess of temperature can slow down the photosynthetic activity of the tree, slowing down the oil biosynthesis process and also causing a loss of fruit size. Therefore, the optimum temperature is between 20 and 30 ºC. Temperatures below 20 degrees or above 30, suppose a decrease in photosynthesis, while above 35 ºC the CO2 and photosynthetic uptake processes would suffer a drastic fall. To improve the lighting and temperature conditions we can influence through pruning, always opting for lobed shapes that allow greater interception of radiation, with respect to globose shapes (Figure 6).
The technical or design factors of the plantation are not a limitation in traditional or intensive olive grove plantations since the plantation frames are wide enough to allow the illumination of the four faces of the olive tree and its upper part. However, in super-intensive or olive grove systems in a hedge, a series of design considerations must be taken into account to improve lighting to maximize the productive potential of the hedge. When the first olive groves were established in hedgerows, it was observed that some were poorly designed and managed, and that the high size of the hedges and the reduced distance between them, prevented light from reaching the low areas, so they defoliated and production was located in ever higher areas. These observations made it possible to establish a series of tests to determine the optimal characteristics that a hedge should have (Figure 7), with porosity of 20%, to achieve maximum production: the height of vegetation of the hedge (Alt) must be less than or equal to the free distance between the hedges (d), therefore Alt / d <1 (Connor and Gómez del Campo, 2013). If the porosity is reduced, the free distance between hedges (d) should be increased or decreased in height (Alt) and width (a).
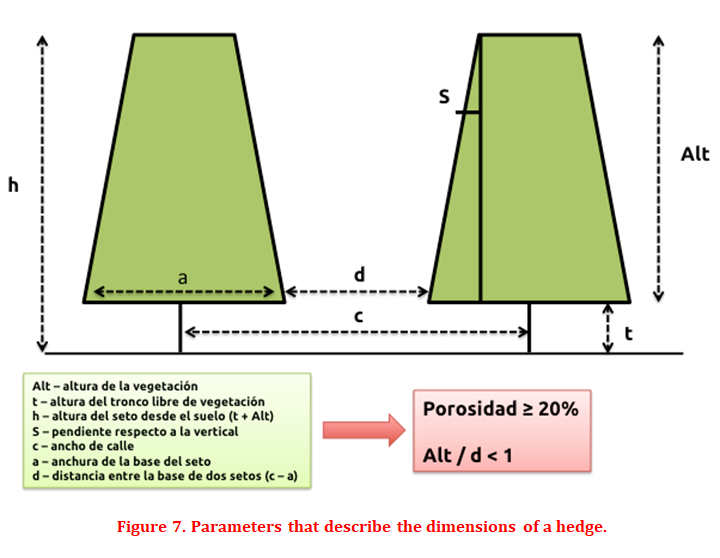
Continuing with the influence of the plantation design in the hedgerow olive grove, the oil production per hectare increases as the width of the street is reduced, since we are increasing the number of trees per hectare. However, oil production per tree is greater when the lane width increases, something that is more accentuated with an EW orientation with respect to NS, where the influence of the lane orientation has little effect on oil production. per tree (Trentacoste et al, 2015a). Other tests carried out with different orientations where the hedges have been designed with a street width of 4 meters, a porosity of 20% and an Alt / d ratio = 0.69 indicate that the hedges with EO orientation had a similar production of quantity of oil than the NS hedges, even slightly above when the autumns were rainy and, therefore, scarce radiation (Gómez del Campo et al., 2009). In summary, when the hedge is well designed, the effect of orientation on oil production is small (Trentacoste et al., 2015b)
The weather has a great influence on the olive harvest and also on the formation of oil. The lack of water in the crop to cover its needs, called water stress, directly affects photosynthesis. The response of the plant generally translates into a longer stomatal closure than in normal conditions in order to reduce transpiration (with the consequent saving of water), which directly affects gas exchange, reducing the rate of photosynthesis and therefore the formation of assimilates. The duration time and the moment of the cycle in which the hydric stress occurs determine the vegetative and productive response of the plant (Figure 8).
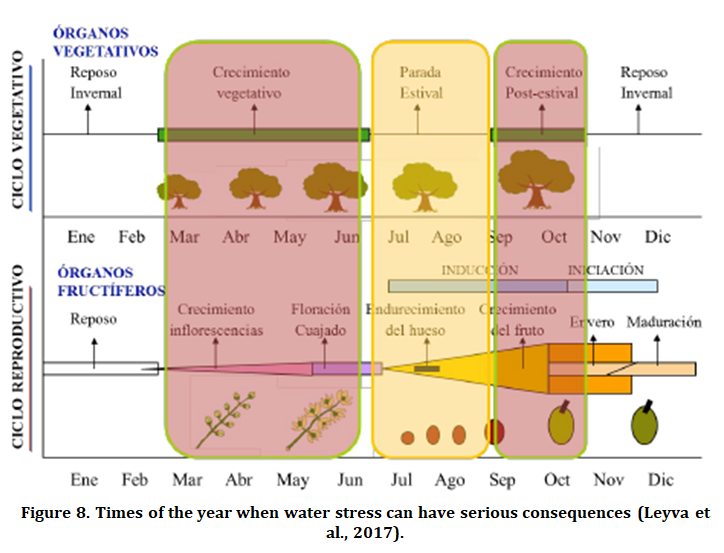
Spring is the time of year when processes of great relevance take place in cultivation. The formation of inflorescences concur, with the subsequent flowering and fruit setting until the bone hardens. Most of the shoot growth to give also happens. rise to the fruitful positions of the following year. Under normal conditions, and with relatively deep soils and high water storage capacity, winter rain is sufficient to avoid spring water stress. However, in some olive groves in years with very dry winters there can be serious problems (poor flower quality, poor fruit setting and poor shoot growth).
Summer is the time of year when the first phases of fruit growth occur. Some conditions of water stress during lipid biosynthesis cause a reduction in the oil formation capacity (Lavee, 1991), as well as a slowdown in fruit growth. In the event that the olive has oil production as its final destination, certain water stress can be assumed, which causes a reduction in the size of the fruit. Depending on the degree of stress and its duration, the loss of production can be significant.
Autumn is a period of great lipogenic activity (oil formation) and fruit development (size) and is generally in our conditions, the period most sensitive to water stress (Figure 9). At this time, it is essential to cover the water needs of the crop through irrigation, in the event that the rain is insufficient, in order to obtain as much oil as possible (Leyva et al., 2017).

Importance of controlling the water deficit during lipogenesis:
- In case of reaching high values of water deficit, the growth of the fruit would slow down and even stop in case of being severe and prolonged in time.
- Increases in irrigation doses translate into increases in production.
- Autumn rains are essential for good oil production (Leyva et al., 2017).
- The application of irrigation reduces the concentration of components related to the quality of the oil (chlorophylls, polyphenols, carotenes and pigments (Amilo et al., 2019).
Conditions of hydric stress during lipid biosynthesis and abnormally low pulp/bone ratio causes a reduction in the capacity of oil formation and thus its fat content. There is a negative correlation between the production, the size of the fruit and the fat content of the pulp. Just like that, seasons with large crops often give fruits of smaller size and lower fat content than those obtained in seasons of low production (Lavee and Wodner, 1991).
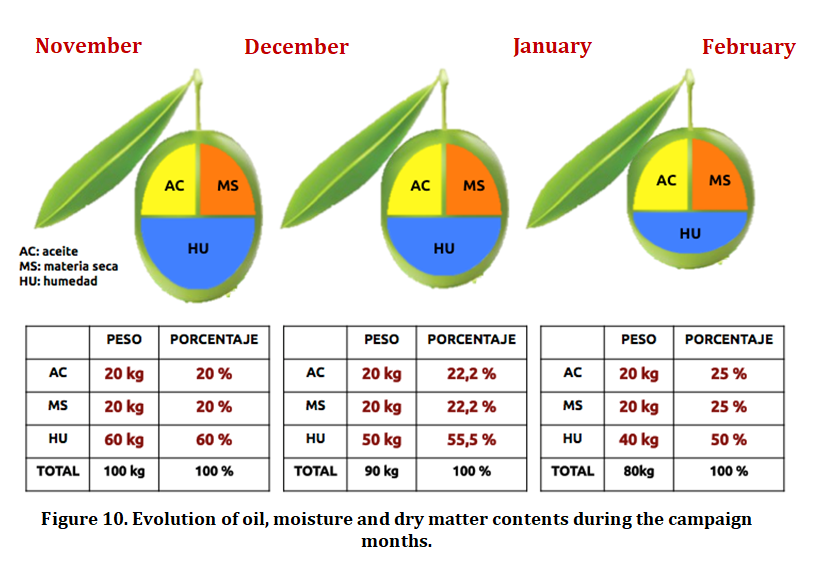
Water is the other major component of the fruit along with the oil and decreases during the ripening process, showing significant variations as a result of climatic conditions from mid-November (rainfall and frost regime). In order to eliminate water interference when expressing the fat content of the fruit and to accurately indicate the completion of the oil formation phase, it is advisable to express the fat content on dry matter, since this parameter remains constant once the synthesis of lipid stops, unlike when it is expressed on fresh weight, since this sample increases until very late periods (late January), mainly due to the decrease in the humidity of the fruit (Figure 10). The fat content on dry matter remains constant from the moment the lipid synthesis is completed, in other words, the oil formation stops between mid-November and early December, depending on the variety considered (Beltrán et al. , 2017).
Finally, within the external factors, nutrition affects in an important way the biosynthesis of fatty acids. The olive tree is a rustic plant, capable of being a vegetable and producing fruit even under adverse environmental conditions for many other species. Like any perennial plant, it has organs for reserving nutrients that are easily reused. For all these reasons, the nutritional needs of the olive grove are lower than those of other crops. Nitrogen (N) is the nutritive element that is required in greater quantities by plants, including the olive tree, which is why it has traditionally constituted the fertilization of the olive grove. In dry conditions, the greatest nutritional problem is the deficiency in potassium (K), which is aggravated in case of a high harvest. In limestone soils, in addition to potassium, cases of iron (Fe) and possibly boron (B) deficiency can be found, and in acid soils deficiencies in calcium (Ca) can be expected. These are the nutritional imbalances that can affect most of the olive grove and that, ultimately, should be monitored by carrying out the corresponding analyzes. However, these imbalances will hardly appear concentrated in the same plantation (Fernández Escobar, 2017).
To favor the synthesis of fatty acids it is necessary to maintain a good nutritional level of the olive tree, focused mainly on the elements to be contributed during this stage in order to be able to express the greatest capabilities of the cultivated variety in terms of fat yield.
Nitrogen is the major nutrient component of plants, which is why it is usually the most commonly used mineral element in fertilization programs. In the case of diagnosed deficiency, the symptoms of which are characterized by a generalized loss of chlorophyll that gives rise to nonspecific chlorosis in the limbus (Figure 11), a dose of nitrogen should be used to correct it depending on the size of the tree, its level. production and culture medium, and will have to be adjusted by carrying out periodic foliar analyzes that, correctly interpreted, will indicate the need to increase or reduce the applied doses. Due to the positive nitrogen balance, that is, the inputs into the olive grove are higher than the outputs, in most olive groves it is difficult to find situations of deficiency of this nutrient element.

Few studies have been carried out historically to show the possible adverse effects of an excess of nitrogen fertilization in the plant, although a greater susceptibility of trees to the action of pests and diseases and a lower tolerance to cold have been cited, although with certain controversies between authors. More recently, work has been carried out in this regard, it has been proven that excess nitrogen fertilization in olive trees increases the accumulation of nitrogen in the fruit and causes a significant decrease in the quality of the oil (Fernández Escobar et al., 2006). A delay in fruit ripening has also been detected, which usually causes a decrease in fat yield (Fernández Escobar et al., 2014). Nitrogen concentrations in leaves above 1.7% have caused these effects, which is why this value has been established as a level of toxicity.
What had been observed historically in the olive grove was the lack of response of the olive tree to excessive or unnecessary applications of nitrogen and that the annual maintenance fertilizer with nitrogen is meaningless in the olive grove, and that only the application of nitrogen fits when the concentration in leaves indicates a deficiency situation. For this reason, foliar analysis is a useful tool for planning the annual fertilization of an olive grove.
Potassium is the element that is extracted the most in the crop at harvest. This means that it is a very important element in the nutrition of the olive tree and that it is magnified due to the influence that the culture medium has on the availability of potassium from the tree. In fact, it constitutes the main nutritional problem of the dry olive grove, with great consequences on the crop since potassium intervenes in the mechanism of closure and opening of the stomata. Located in the cytoplasm inside the plant and helps in keeping it turgid. In conditions of deficiency, the stomatic closure is not complete and the tree continues to lose water through perspiration, and may show symptoms of dehydration. Well-nourished trees, by contrast, will easily tolerate drought conditions by completely closing stomata at times of high radiation.
Deficiencies, or low potassium levels, are popular in large part of the olive grove. Deficient trees show apical or lateral leaf necrosis and branch defoliation; In seasons of harvest, the fruits are wrinkled and smaller than normal. These deficiencies manifest themselves more intensely in dry olive grove and in dry years, since the low soil humidity limits the diffusion of potassium ion (K+) in the soil dissolution and prevents its absorption by the roots. Deficiencies are also frequent in soils with low clay contents, as the buffering power of the soil is lower and, consequently, the K+ available for the tree.

The causes for potassium deficiency are diverse. In addition to the lack of soil humidity in rainfed plantations, stands out the true nature of cultivated varieties and the interactions with calcium (Ca2+) and magnesium (Mg2+) ions, which in limestone soils can explain the generalized deficiencies of this element.
Olive groves with potassium deficiency are difficult to amend, since the potassium, as fertilizer, is absorbed in smaller quantities in deficient and with water stress trees, even if it is applied via foliar (Restrepo et al., 2008a). Therefore, it is convenient to annually monitor the levels of potassium in leaves and apply this element when low values are reached, before reaching deficiency. In soil applications, it should be considered that potassium, unlike nitrogen, has low mobility, particularly if the clay content is higher. This means that potassium stays on the soil surface, unless it is located in the vicinity of the radical system (Fernández-Escobar, 2017). For this reason, in dry land it is recommended 2 to 4 foliar applications at 1-2% of K+ depending on the nutritional status of the tree, although repetition is usually necessary in successive seasons until the levels of K+ in leaves are raised to its appropriate level. That is to say, foliar applications must be made both in seasons of high load and in seasons of significant alternation (vecería – masting).
Young leaves absorb potassium to a greater extent compared to adult leaves, so applications of this nutrient in spring are very effective (Restrepo-Díaz et al., 2009). On the other hand, from the period of hardening of the bone, when lipogenesis begins, K+ treatments are of great interest for two reasons. On the one hand, proper nutrition of this element improves the stomatic behavior of the plant, which will close the stomata at increased radiation during the summer, thus reducing the water stress of the plant (lower perspiration rate). On the other hand, during the fatty acid synthesis phase, the fruit is the largest potassium drain, whose levels must be adequate to improve the fat yield of the fruit and increase its size. Applications after the veraison (envero) will not improve neither the size nor the fat content of the olive.
In addition to this, potassium is essential for the following physiological functions:
- For the synthesis of carbohydrates or formation and transformation of starch, as well as in the manufacture of albumins.
- It is involved in nitrogen metabolism and protein synthesis.
- It controls the activities of several essential mineral elements.
- It contributes in the neutralization of physiologically important organic acids. These acids tend to lower the pH of the cell juice, which are neutralized by K+ (Baeyens, 1970).
- Acts as a trigger of several enzymes, which catalyze phosphorylation and on the contrary inhibits those of respiration.
- Promotes growth in the meristematic tissues.
Much of the Spanish olive grove is found on calcareous soils with an alkaline reaction, which has made it difficult to find calcium deficiencies in this olive grove. It would be more likely to find calcium deficiencies in acidic soils, manifesting at the plant level through a reduction in growth and in severe cases affecting the consistency of the olive pulp, which can cause quality problems in table olives. In these cases, the soil should be limed using calcium carbonate or calcium oxide. On the contrary, excess calcium can cause deficiencies of potassium and magnesium, since these three ions interact with each other in the complex of soil change. In some cases there may be a calcium deficiency not linked to the pH of the soil. In these cases, foliar applications with calcium complexed with organic acids would be a good short-term solution.
Magnesium is an element that is usually found in significant quantities in the dissolution of the soil with a behavior in the same similar to that of calcium, so the deficiency of this element in the olive grove is very rare. In the case of acidic soil, deficiencies could be found that should be corrected by trying to neutralize the acidity, as in the case of calcium. It must be taken into account that magnesium deficiencies can sometimes be induced by high concentrations of potassium, calcium and ammonium, since these ions compete in the soil solution. If the ratio K of change / Mg of change is greater than 1, these deficiencies can be expected. The symptoms of magnesium deficiency manifest as chlorosis in the oldest or basal leaves, causing a significant loss of photosynthetic activity. As in the case of calcium, foliar applications of chelated magnesium would prove to be very effective in the short term.
Iron deficiency, known as iron chlorosis, is a nutritional imbalance that can affect olive groves established in very limestone soils, with a high pH. In this medium, the ionic forms of iron are poorly soluble and are not available to plants even though they are present in sufficient quantities in the soil. Trees affected by iron chlorosis show characteristic symptoms of leaf chlorosis (in this case, younger leaves) characterized by a yellowness of variable intensity in the blade but keeping the veins green, accompanied by a decrease in the size of the apical leaves. , a small growth of the shoots and a decrease in production (Figure 14). These symptoms are the means of diagnosis of the deficiency, since the foliar analysis is not useful in this case, since the iron accumulates in the leaves even in situations of deficiency. Iron deficiency is also related to conditions of poor soil aeration, since it increases the concentration of bicarbonate anion in the soil solution, aggravating iron chlorosis. Therefore, waterlogging conditions must be avoided in limestone soils. Correction of iron chlorosis is difficult and expensive. The best solution for new plantations is the choice of a variety tolerant to this anomaly. In established olive groves, the remedy goes through the annual application of iron chelates to the soil, which allow the disposal of iron to the plant for a moderately long time compared to other products.

The amounts of manganese and zinc required by the olive tree are even lower than those of other elements and it tends to take them easily from the soil solution. Relatively little is known about the relationship of these elements with the olive tree, since they are usually found in leaves at relatively adequate levels, so the possible deficiencies must be local in scope. Manganese intervenes in biological processes such as photosynthesis, respiration, nitrogen assimilation, etc., it is related to protein metabolism and enzymatic activation, it also acts on carbohydrate and fatty acid metabolism, phosphorylation reactions and formation of nucleic acids. For its part, zinc is a very important micronutrient since it performs functions as an enzyme activator in the synthesis of certain proteins, although its main function is related to the regulation of growth and stem elongation, since it has a direct relationship in synthesis of auxins. Organic amendments applied to the soil can improve the availability, mobility and absorption of these micronutrients, while foliar applications of chelates can also be an effective solution to improve the levels of these nutrients in the olive tree.
The olive tree is a plant that is considered to have high boron requirements and, in fact, it is more tolerant of an excess of boron in the soil solution than other fruit species. Boron availability by plants decreases under drought conditions and as soil pH increases, particularly in calcareous soils. These environmental conditions are frequent in olive groves. Boron deficiency is manifested by apical and marginal chlorosis in leaves that end up drying and showing a chlorotic zone between the dry part and the still green part of the leaf. Shoot defoliation is also known, giving rise to the so-called “witch’s brooms” and deformations in the fruits. It is very important not to confuse boron deficiency with potassium deficiency (Figure 15), hence the importance of performing a foliar analysis to diagnose this type of nutritional problems. In fact, an additional problem is that boron applied in excess is a toxic ion, which can even lead to the death of olive plants, particularly young ones (Benlloch et al., 1991).

Phosphorus is an important element in the fertilization of crops, related to the formation of root tissue and flowering, although it is crucial for the plant for other reasons. It is an essential element of difficult dosage and is required in notably smaller amounts than nitrogen. It is absorbed in anionic means, usually as orthophosphate ion (H2PO4-), depending on the pH from the soil. It is present in constitution compounds (phosphate sugars, nucleic acids, phospholipids and coenzymes) and in energy transport compounds (ATP, NADP, FAD) (Westerman, 1990). Its main physiological role consists in the phosphorylation of organic substances. Phosphoric acid temporarily combines with a carbonyl, enolic or nitric group to form an energy-rich compound: adenosine triphosphate (ATP) which, decomposing in ADP, releases this energy which is used in metabolic processes (Baeyens, 1970). These metabolic processes create chemical reactions that directly intervene in the survival, growth and reproduction of plants, such as photosynthesis, respiration, solute transport, translocation, protein synthesis, nutrient assimilation, differentiation of tissues, and in the formation of carbohydrates, lipids and proteins that intervene in these processes or are a structural part of plants.
It is likely that only trees grown on soils with very poor phosphorus will have poor levels of leaf concentrations. Symptoms of deficiency begin in the lower leaves, that is, in the older ones, turning from a dull dark green color to a characteristic reddish or purple color and which completely dries the leaves. The number of sprouts decreases, with the presence of thin and short stems with small leaves. Root development and regeneration, flowering and fruit setting are also reduced.

RECOMMENDATION
Therefore, from the Technical Department of Cultifort, we recommend making interventions during the fruiting period with specific nutritional products, adapted to each phenological state, in order to improve the quality of the fruits. In the case that we deal with in this article, the olive grove, whether rainfed or irrigated, we offer you different formulations specially developed for the ripening and fattening stages of the fruits.

MACROFOL ROJO PLUS is a soluble NPK, with a 15-5-30 balance formulated with magnesium and micronutrients. Its composition is designed to favor the development, fattening, maturation and consistency of the fruits, increasing the storage of carbohydrates and proteins in them. It also supposes an ideal supply of nitrogen, with a concentration that does not negatively affect the ripening of the fruits, during the phenological stages in which its application is most recommended. MACROFOL ROJO PLUS is a highly soluble product, very stable and that behaves well against mixtures with other products on the market, does not form lumps during dissolution, has a slightly acidic pH and, best of all, it is a chlorine-free fertilizer.

CULTIFORT K and CULTINEUTRAL K are two liquid potassium formulations of high richness and free of chlorine. They are designed to favor the fruit fattening and ripening process, increasing their size and uniformity, increasing the synthesis and accumulation of sugars and improving their color and firmness. Thanks to the technology of their formulation, they are quickly assimilable products, with high absorption, mobility and translocation within the plant. They are similar in terms of richness and their differences lie mainly in pH, 9 in CULTIFORT K and 6 in CULTINEUTRAL K, and in density, 1.5 and 1.24kg / l, respectively.

In the Cultifort catalog we also have a complete range of amendments and deficiency correctors. Solutions for all needs and / or deficiencies: calcium, magnesium, iron, manganese, zinc, boron, micronutrient mix, etc., all of them formulated with high quality raw materials, chelated by different agents or complexed with organic acids to improve its assimilation. In addition, our formulas are “zero residue” and many of them are certified for use as inputs in Organic Agriculture.

REFERENCIAS
Baeyens, J., 1970. Nutrición de las plantas de cultivo. Madrid (España). Ediciones Lemos.
Beltrán, G., Uceda., M., Hermoso, M. y Frías, L., 2017. El Cultivo del Olivo. Maduración. Madrid (España). Ediciones Mundi-Prensa. 198-203.
Benlloch, M., Arboleda, F., Barranco, D., Fernández Escobar, R., 1991. Response of young olive trees to sodium and boron excess in irrigation water. HortScience, 26: 867-870.
Connor, D. J., & Gomez del Campo, M., 2013. Simulation of oil productivity and quality of N-S oriented olive hedgerow orchards in response to structure and interception of radiation. Scientia Horticulturae , 150: 92–99.
Emilov H., Pérez H., Cuevas A. y Sastre B., 2019. Estudio comparativo de seis variedades de olivo en secano y regadío cultivadas en la Comunidad de Madrid. Actas de Horticultura 84. VI Jornada Nacional del Grupo de Olivicultura. Sociedad Española de Ciencias Hortícolas.
Fernández Escobar R., 2017. El Cultivo del Olivo. Fertilización. Ed. Mundi-Prensa. Madrid. 419-460.
Fernández Escobar R., Beltrán G., Sánchez Zamora M.A., García Novelo J.M., Aguilera M.P., Uceda M., 2006. Olive oil quality decreases with nitrogen over-fertilization. Hort Science, 41: 215-219.
Fernández Escobar R., Braz Frade R., Beltrán G., López Campayo M., 2014. Effect of nitrogen fertilization on fruit maturation of olive trees. Acta Horticulturae, 1057: 101-105.
Frías, L., García- Ortiz, A., Hermoso, M., Jiménez, A., Llavera, M.P., Morales, J., Ruano, T. y Uceda, M., 1991. Analistas de laboratorio de almazaras. Series Apuntes 6/91. Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible. Junta de Andalucía.
García-Martos, J.M. y Mancha, M., 1992. Evolución de la biosíntesis de lípidos durante la maduración de las variedades de aceituna “Picual” y “Gordal”. Grasas y Aceites, 43(5): 277-280.
Gomez del campo M., Centeno A., Connor D.J., 2009. Yield determination in olive hedgerow orchards I. Yield and profiles of yield components in north-south and east-west oriented hedgerows. Crop and Pasture Science 60: 434-442.
Gutiérrez, F., Jiménez, B., Ruiz, A. y Albi, M.A., 1999. Effect of olive ripeness on the oxidative stability of vrgin olive oil extracted from varieties “Picual” and “Hojiblanca” on the different components involved. . Journal of Agriculture and Food Chemistry, 47: 121-127.
Lavee, S., 1986. C.R.C. Olive. Handbook of Fruit Set and Development. Pp. 261-274. Monselise, S.P. Ed., C.R.C. Press, Florida.
Lavee, S. y Wodner, M., 1991. Factors affecting the nature of soil accumulation in fruit of olive (Olea europea L.) cultivars. Journal of Horticultural Science 66(5): 583-591.
Leyva A., Hidalgo J.C., Vega V., Pérez D., Hidalgo J., 2017. El estrés hídrico y la formación de aceite de oliva. IFAPA. Consejería de Agricultura, Pesca y Desarrollo Rural. Junta de Andalucía.
Minguez, M.I., Rejano, J.L., Gandul, B., Higinio, A. y Garrido, J., 1991. Colour pigment correlation in virgin olive oil. Journal of the American Oil Chemists’ Society, 68: 669-671.
Molina de la Rosa, J.L., Jiménez, B., Ruiz, F., García, F., López, F., Salmerón, E., 2010. Agronomía y Poda de Olivar. Consejería de Agricultura y Pesca. Junta de Andalucía.
Navas López J.F, 2019. Mejora de olivo para adaptación a diferentes condiciones ambientales y sistemas de cultivo. Tesis Doctoral. Universidad de Córdoba. España.
Pérez Camino, M.C. y Cert, A., 1999. Quantitative determination of hidroxypentaciclic trterpene aceids in vegetable olive oils. Journal of Agriculture and Food Chemistry, 47: 1558-1562.
Rallo, L. y Cuevas, J., 2017. El Cultivo del Olivo. Fructificación y Producción. Madrid (España). Ediciones Mundi-Prensa. 162-167.
Restrepo, H., Benlloch, M. y Fernández-Escobar, R., 2008a. Plant wáter stress and K+ starvation reduce absorption of foliar applied K+ by olive leaves. Scientia Horticulture, 116: 409-413.
Restrepo, H. , Benlloch, M. y Fernández-Escobar, R., 2009. Leaf K accumulation in olive plants related to nutritional K status, leaf age and foliar application of K salts. Journal of Plant Nutrition, 32(7): 1108-1121.
Trentacoste, E. R., Connor, D. J., & Gomez-del-Campo, M., 2015a. Effect of row spacing on vegetative structure, fruit characteristics and oil productivity of N-S and E-W oriented olive hedgerows. Scientia Horticulturae , 193 , 240–248.
Trentacoste E.R., Connor D.J., Gómez del Campo M., 2015b. Effect of olive hedgerow otientation on vegetative growth, fruit characteristics and productivity. Scientia Horticulturae, 192: 60-69.
Vega, V., Hidalgo, J.C., Pariente, N., Martín, L., Hidalgo, J., Leyva, A., 2018. Corrección de Clorosis Férrica en Olivar mediante Quelatos. IFAPA. Consejería de Agricultura, Pesca y Desarrollo Rural. Junta de Andalucía.
Westerman, R.L., 1990. Soil Testing and Plant Analysis. Soil Science Society of America Book Series Nº 3. Madison, Wisconsin (USA). Editorial Committee.

