BACKGROUND
Avocado exhibits rhythmic growth, with two or more shoots per year, alternating with short periods of rest. Growth fluxes can be vegetative or reproductive. Vegetative sprouting allows the tree to increase or renew its photosynthetic capacity and also to generate new buds that, subsequently, originate reproductive flows. Reproductive flows are those buds or growths that give rise to flowering, and can be indeterminate or determinate (Alcaraz et al., 2013). The indeterminate ones are constituted by the inflorescence and its corresponding leaves, while the determinate ones lack leaves.

A. Indeterminate inflorescences; B. Determined inflorescences de P. americana cv. Hass. Sources: Alcaraz et al. (2013).
The avocado grows mainly in three climatic zones: cold, semi-arid climates with predominant rainfall in winter (California, Chile, Israel); humid subtropical climates with predominant rainfall in summer (eastern Australia, Mexico and South Africa), and tropical and semi-tropical climates with predominant rainfall in summer (Brazil, Florida, Indonesia). It is further divided into three ecological races: Mexican, Guatemalan and Antillean. Cultivars within each race generally show similar responses to climatic and edaphic conditions. However, there are differences between races and between cultivars with respect to their adaptability to environmental conditions (Whiley and Shaffer, 1994), as in the case of cv. ‘Hass`, a hybrid between the Mexican and Guatemalan races, which has intermediate characteristics between the two.
Broadly speaking, the ideal temperature conditions for this species are around 25-30ºC for diurnal crops, and between 15 and 20ºC for nocturnal crops. Temperatures above 36ºC cause serious damage, particularly in fertilization and fruit set, and it is important that a cold period (around 10ºC) occurs in winter to stimulate flower induction (Galán, 1990).
Photosynthetic activity is an indicator of the growth and productivity of a crop. Growth and yield are strongly dependent on carbohydrate partitioning. Increasing production in subtropical species with terminal polyaxial fruiting, as is the case of avocado, poses a challenge for agronomic management, since the tree has a natural tendency to vegetative growth that results in a greater allocation of dry matter to it to the detriment of the development of reproductive organs (Wolstenholme, 1990). Environmental factors such as light, temperature and CO2 concentration affect photosynthesis, respiration and carbohydrate partitioning.
The partitioning of photoassimilates is regulated by source-sink interactions. Sources are exporters and sinks are net importers of photoassimilates (Ho, 1988). The order of priority of demand is a function of growth rate (sink activity) and sink size (number of fruits). The order, generally, is: seed > fruit pulp = shoot and leaf apices > cambium > roots > roots > storage tissues (Wolstenholme, 1990). Young leaves, while expanding, are strong sinks that compete with other demanding organs of the plant until they reach their final size, at which time they become net exporters (Ho, 1988).
The availability of incident light is probably the factor that exerts the greatest influence on photosynthesis in a fruit orchard. In avocado, toward the end of spring shoot growth, light transmission to the fruiting zone is reduced to 40% of full illumination, and at distances of 0.5 and 1.0 m into the canopy from the fruiting zone, it is reduced to 14% and 10%, respectively. Towards the end of summer shoot growth, light transmission to the fruiting zone at full illumination has decreased to 13%, and at the inner points (0.5 and 1.0 m) to 9.7% and 6.3%, respectively (Whiley et al., 1992).
The intensity and duration of illumination are determinants of flowering (Coutanceau, 1964), and it is widely known that flowering and fruiting are less abundant in shade than in full light (Meyer, 1960). When illumination is low in relation to its requirements, vegetative growth is reduced, both in number and length of shoots, as well as in leaf size, resulting in reduced tree development and photosynthetic activity. Thus, in the interior of the tree numerous branches originate and the density of external branches reduces the illumination, therefore, the flowering inside the tree; only the external part of the canopy with adequate illumination presents satisfactory flowering (Coutanceau, 1964).
PHENOLOGY AND DEVELOPMENT
The avocado is characterized by a monopodial rhythmic growth, that is, with a growth of a terminal vegetative bud from the central axis of each shoot that remains and continues its development year after year, and is an example of the Rauh architectural model, one of the most frequent in temperate and tropical zones.
The trunk forms branches that are morphogenetically identical to the trunk and the flowers originate laterally without having an effect on shoot growth, although in some shoots there are flowers in terminal position, the growth being subsequently sympodial (Thorp and Sedgley, 1992).
Buds can be axillary or apical. The tree grows mainly from the apical buds, because axillary buds are shed or remain dormant (Calabrese, 1992). The vigor of full tree growth and fruit production depend on the timing and extent of phenological events, which is controlled by the availability of carbon and energy and their distribution (Wolstenholme and Whiley, 1989) in response to environmental conditions (Scholefield et al., 1985). Leaves require about 40 days from budding to the transition from sink to source (Whiley, 1990). During this period they may compete for photoassimilates with developing fruit (Cutting and Bower, 1990).
Avocado throughout the year may have one or more vegetative cycles followed by a period of root growth. Roots begin to grow when the first vegetative growth begins to decline. Subsequently, a second period of vegetative growth begins, thus reestablishing the balance between a vegetative and a root growth phase (Calabrese, 1992).

Avocado is a fruit tree with a marked effect of crop alternation, where optimal climatic conditions for fruit set will produce a year of high yield, which will be followed by a year of low production and, on the contrary, a year of climatic conditions that produce a high abscission of flowers and/or fruit, will give rise to a year of low production and, subsequently, the following year, increase flowering and thus the harvest (Lovatt, 2004).
Flowering and fruit growth use large amounts of carbohydrates, so a deficit of carbohydrates would limit these processes and result in a lean year. In other words, a high fruit load would be the main cause of reduced carbohydrate accumulation during the fall and winter, resulting in low flowering and a large vegetative development the following year. Conversely, during a low production year, trees would accumulate higher levels of carbohydrates during the fall and winter, resulting in intense flowering (Paz Vega, 1997).

Scholefield et al. (1985) state that in autumn the concentration of carbohydrates is minimal, at the same time that there is a decrease in vegetative activity, which translates into less competition between vegetative and reproductive development. These authors point out that, in accordance with this, floral initiation in avocado takes place when carbohydrate levels are minimal, thus there is a close relationship between carbohydrate levels and floral initiation. Low carbohydrate levels could be the cause of vegetative growth arrest and this factor could be the major determinant of floral initiation.
In avocado, vegetative growth occurs just when the levels of reserves in the branches are very low. The highest levels of starch reserves in the wood are found in winter, coinciding with the cessation of vegetative growth. These reserves fall rapidly during flowering, to be at their lowest levels during the summer, when fruit abscission occurs (Whiley and Wolstenholme, 1990). This accumulation of starch in winter determines the maintenance of the fruit on the tree.
In indeterminate inflorescences, leaves compete for photoassimilates with flowers and developing fruit until they reach 2/3 of their total expansion (Whiley, 1990), and once fruit begin to develop, the direction of photoassimilate transport shifts in favor of fruit growth (Ho, 1992).

An additional fact used to support the hypothesis that carbohydrate availability could be the regulator in the cycle of productive alternation is the high oil content of the avocado, which is energetically very costly to accumulate. Compared to other fruits, such as apples, oranges and bananas, the amounts of sugars they store are much lower, but their high oil content could explain the drastic reduction of carbohydrate reserves in a year of high production.
It is also accepted that the productive alternation is related to the role of growth regulators (PGRs) produced by the seed in inhibiting flowering in the following year (Paz Vega, 1997). In avocado, gibberellic acid produced by the endocarp of the fruit has been related to decreased flowering and fruit production (Martens et al., 1994). The role of carbohydrate reserves is, under this point of view, questioned (Liu et al., 1999).
Scholefield et al. (1985) mention that the cessation of vegetative growth is related to a decrease in the production of gibberellins, allowing the initiation of floral buds, and, on the other hand, fruits are characterized by their intense metabolic demand.
In general, we can conclude that many factors make it difficult to study crop alternation and its relationship with vegetative growth, for example, nutritional, hormonal and climatic factors and other factors derived from endogenous reactions involved in competition between growing organs. What we can do is to prevent its effect from being accentuated by acting on those factors that we can control, such as nutritional factors.
Finally, it should be noted that starch and soluble sugars are the predominant carbohydrate reserves to provide energy for growth (Dey and Dixon, 1985).
RECOMMENDATION
For all these reasons, from the technical department of Cultifort, it is recommended to work nutritionally during the most demanding stages of the avocado, so that vegetative growth and flowering and fruit set are only affected by those factors that are beyond human control.
For more information on avocado cultivation: advice from an expert another article you may be interested in preventive treatments against fruit drop.
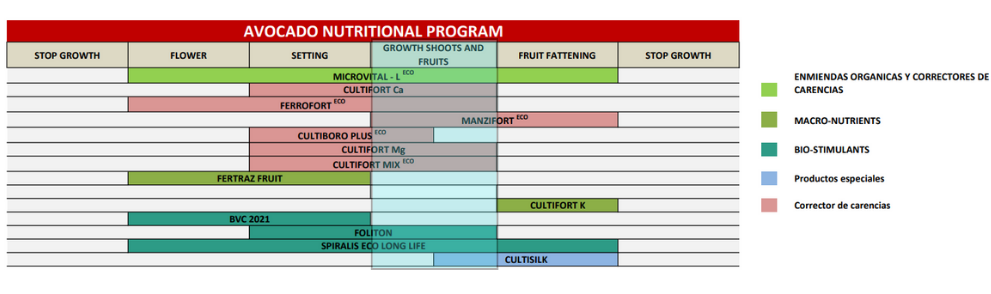
Cultifort offers a wide range of biostimulants, deficiency correctors, organic amendments and other special products to meet the demands of avocado in each of its phenological stages.
In the current phenological stage, shoot and fruit growth, it must be taken into account that there is a high competition for photoassimilates or carbohydrates between the growing fruit and the young leaves and shoots. This competition can also cause an increase in physiological fruit drop if not adequately controlled. It is therefore highly recommended to correct any nutritional deficiencies and to work on biostimulation at this critical stage.
As a basis for this period, it is recommended to improve edaphic conditions, since we have already seen that for vegetative growth to be efficient, it must be linked to good root development. MICROVITAL – L, a liquid formulation rich in organic acids, flavonoid molecules, magnesium and micronutrients, is a biological soil activator of vegetable origin which, in addition to improving the physicochemical parameters, acts as a prebiotic product, stimulating the growth and development of the edaphic microfauna. These effects are achieved thanks to:
– Organic acids, in forms directly assimilated by the plant (as opposed to other organic acids)
– and flavonoid molecules, which, as polyphenols, have antioxidant properties, helping the plant to remain photosynthetically more active, delaying leaf senescence and exerting a protective action against different types of abiotic stress, such as ultraviolet radiation. They also have iron and other metal chelation properties, so the use of MICROVITAL – L contributes to the mobilization of nutrients present in the soil.
As if that were not enough, MICROVITAL – L corrects and prevents deficiencies of magnesium, boron, iron, manganese and zinc. The plant remains greener, metabolically more active, with a better development… in general, healthier and stronger.
Within the range of deficiency correctors, Cultifort has all kinds of nutritional solutions: magnesium, calcium, iron, boron, zinc, manganese, molybdenum, etc., formulating all of them with the most efficient technology for their correct and maximum assimilation, using different chelates and complexing agents, together with carbohydrates, organic polyacids, polycarboxylic acids, polysaccharides and reducing sugars.
 At the biostimulation level, FOLITON is a biostimulant with a high content of proteinogenic L-amino acids, both free and combined in the form of peptides and polypeptides, which allow avocado to save energy in the formation of proteins, especially when there are high nutritional requirements. It is a liquid formulation of rapid assimilation and translocation in the plant. Its application represents a strong stimulation of plant metabolism at times when increased vegetative activity is required. The joint action of the components of its formulation favors the synthesis of proteins and carbohydrates, promotes the beginning of the plant’s physiological activity and stimulates the formation of leaves and flowers, reducing the competitive relationship between them.
At the biostimulation level, FOLITON is a biostimulant with a high content of proteinogenic L-amino acids, both free and combined in the form of peptides and polypeptides, which allow avocado to save energy in the formation of proteins, especially when there are high nutritional requirements. It is a liquid formulation of rapid assimilation and translocation in the plant. Its application represents a strong stimulation of plant metabolism at times when increased vegetative activity is required. The joint action of the components of its formulation favors the synthesis of proteins and carbohydrates, promotes the beginning of the plant’s physiological activity and stimulates the formation of leaves and flowers, reducing the competitive relationship between them.
SPIRALIS Long Life is a formulation of special organic acids together with a complex of selected peptides related to red (Gellidium) and green (Spirulina) microalgae. Its functionality is based on inducing and enhancing the local and systemic endogenous increase of molecules with high defensive capacity (alkaloids, thionins, phytoalexins, PR proteins, etc.). On the other hand, it induces structural changes in plant cell walls at the level of lignification, thus constituting a physical barrier against abiotic stress. It especially stimulates the response levels of Acquired Systemic Resistance (ASR) and Induced Systemic Resistance (ISR) of the plant against fungi and bacteria, among others. Its use is recommended prior to risk situations and/or as long as favorable conditions for disease development are maintained. Its broad spectrum antifungal and antibacterial effect, at a preventive level, and its effect on improving the post-harvest life of the fruit, make SPIRALIS Long Life one of the most complete and technical products in the Cultifort catalog.
But it is also convenient to protect the avocado against different types of stress that may occur during this phase, both at root and foliar level.
OXIFORT is a product that, when applied together with irrigation water, slowly releases oxygen, improving aeration in the root zone, stopping root asphyxia processes and the development of anaerobic microorganisms. It also improves soil structure, making it spongy and allowing air and water to circulate better through the pore space. It increases the yield of fertilization, especially nitrogen fertilization and favors the development of beneficial microorganisms that participate in the transformation and mobilization of soil nutrients. OXIFORT is very important because it prevents stress caused by root asphyxia conditions. Let’s remember that the avocado crop is extremely sensitive to excess soil moisture. Root asphyxia conditions are caused either by poor irrigation management or by excessive rainfall at a time of lower water demand. In any case, there is a period of approximately 90-100 days, after flowering and fruit set, in which this crop is especially sensitive to excess moisture, and can suffer stress due to root asphyxia, which can cause a massive drop of fruit directly. To avoid such situations as much as possible, it is recommended that avocado plantations be carried out in loose soils with a macropore volume greater than 17% (24% is ideal), with good drainage, with a minimum depth, planning well the water outlets of the plot avoiding accumulations in the lower parts and carrying out a good irrigation management, optimizing water inputs and oxygenation of the soil.
CultisilK is a potassium silicate-based formula that also incorporates free amino acids. On the one hand, silicon will create a protective layer on the plant to cushion the thermal regime in the event of excess temperature, also protecting against sun damage; on the other hand, potassium will act as a regulator of good stomatal behavior, helping to maintain cell turgor and thus avoiding excessive water loss through transpiration; and finally, the free amino acids are focused on reinforcing chlorophyll synthesis and activating photosynthesis, avoiding interruption in the synthesis of assimilates and ensuring that the competitive relationships between fruits and growing shoots are not accentuated. As a source of potassium and silicon, it improves plant growth and strengthens its resistance to environmental factors by enhancing self-defenses. Silicon is a structural element that reinforces the cell wall, strengthening the plant’s physical support and protecting it from attack by external agents. It also has synergies with calcium, magnesium and potassium, improving their absorption and transport in the plant.
In short, at Cultifort we offer a wide range of nutritional solutions with specific functions aimed at improving crop yields in an efficient and ecological way. In addition, only “zero residue” products are included in our catalog.
REFERENCES
Alcaraz, M.; Thorp, T. and Hormaza, J. (2013). Phenological growth stages of avocado (Persea americana) accordingto the bbch scale. Scientia Horticulturae, 164, 434-439. https://doi.org/10.1016/j. scienta.2013.09.051.
Calabrese, F. 1992. El aguacate. Madrid. Ediciones Mundi-Prensa. 249 p.
Coutanceau, M. 1964. Fruticultura. España, Ediciones de Occidente. 108p.
Cutting, J.G. and Bower, J.P. 1990. Spring vegetative flush removal: the effect on yield size, fruit mineral composition and quality. South African Avocado Growers´ Association Yearbook 13, 33-34.
Dey, P.M. and Dixon, R.A. 1985. Biochemistry of Storage Carbohydrates in Green Plants, 1−51. Dey, P.M. and Dixon, R.A. (Eds.). Academic Press, London.
Ho, L.C. 1988. Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Ann. Rev. Plant Physiol. 39, pp. 355–378.
Ho, L. C. 1992. Fruit growth and sink strength. In: Fruit set production: Aspects of development, environmental physiology and ecology. Marshall, C., and Grace J. (Eds.). Cambridge Univ. Press. Great Britain. pp: 101-124.
Galán-Sauco, V. 1990. Los frutales tropicales en los subtrópicos (Aguacate-mango-LitchiLogan). España. Ediciones Mundi-Prensa.133p.
Liu, X., Robinson, P., Madore, M., Witney, G. and Arpaia, M. 1999a. Hass avocado carbohydrate fluctuations. I. Growth and phenology. Journal of the American Society for Horticultural Science 124: 671-675.
Lovatt, C. 2004. Eliminating alternate bearing of “Hass” Avocado. Proceeding of the California Avocado Research Symposium, October 30, 2004. University of California, Riverside. California Avocado Commission. Pages 89-95.
Martens, D.A., S. Luck, and W.T. Frankenberger, Jr. 1994. Role of plant growth regulators in vegetative spring flush, flowering and fruit drop in avocado (Persea americana Mill.). Spec. Rpt. Calif. Avocado Dev.Org., Calif. Avocado Soc., Saticoy.
Meyer,B. 1960. Introducción a la fisiología vegetal. Buenos Aires, Eudeba. 57Op.
Paz-Vega, S. 1997. Alternate bearing in the avocado (Persea americana mill). California Avocado Society Yearbook 81: 117-148.
Scholefield, P.B., Sedgley M. and Alexander, D.McE., 1985. Carbohydrate cycling in relation to shoot growth, floral initiation and development and yield in theavocado. Scientia Horticulturae 25, 99-110.
Thorp; T, and Sedgley, M. 1992. Shoot growth and tree architecture in range of avocado cultivars. In: Proceedings of Second World Avocado Congress. California 1992. pp. 237-240.
Whiley, A.W., 1990. CO2 assimilation of developing fruit shoots of cv Hass avocado (Persea americana Mili.) – A preliminary report. S.A. Avocado Growers’ Assn. Yrbk. 13,28-30.
Whiley, A.W. and Schaffer, B. 1994. Avocado. In: Schaffer, B., Anderson, P. (eds.). Handbook of Environmental Physiology of Fruit Crops, Vol 2. Subtropical and Tropical Crops. CRC Press, Boca Raton, Florida. Pp. 3-35.
Whiley A.W., Schaffer, B. and Lara, S.P. 1992. Carbon dioxide exchange of developing avocado (Persea americana Mill.) fruit. Tree Physiology 11, 85-94.
Whiley, A.W., and B.N, Wolstenholme. 1990. Carbohydrate managementin avocado trees for increased production. South African Avocado Growers Association Yearbook 13: 25 – 27.
Wolstenholme B. N. 1990. Resource allocation and vegetative reproductive competition: opportunities for manipulation in evergreen fruit trees. Acta Horticulturae 275:451-459.
Wolstenholme, B.N. and Whiley, A. W. 1989. Ciclos de carbohidratos y fenológicos como herramientas de manejo para los huertos de paltos. Asociación de Cultivadores de Paltos de Sudáfrica. Yrbk. 12: 33-37.






